The poster session will take place Wednesday 17:30-19:30.
There will be drinks and snacks!
Setup: Wednesday from lunch
Take down: Thursday evening
Scroll below table for full abstracts (expandable from title).
| Number | Name | Title |
|---|---|---|
| P01 | Olga Pastushok (Lappeenranta-Lahti University of Technology (LUT), Finland) | Sewage Sludge Valorization in Energy Storage Systems |
| P02 | Lorena Sánchez Moreno (Northvolt AB and Uppsala University, Sweden) | Unraveling Boron’s Influence on Enhancing the Performance of Ni-Rich NMC Cathode Materials for Li-Ion Batteries |
| P03 | David Wragg (Institute for Energy Technology (IFE), Norway) | Mapping Commercial Pouch Cell Electrodes with Synchrotron XRD and XRF |
| P04 | Arjun Muralidharan (Aalto University, Finland) | Comparing Electrochemical Discharge Methods for Lithium-ion Battery Recycling |
| P05 | Olli Sorsa (VTT Technical Research Centre of Finland, Finland) | Battery Upscaling with Sodium-Ion Chemistry: From Active Material Screening to Pouch Cells |
| P06 | Ola Willstrand (RISE Research Institutes of Sweden & Uppsala University, Sweden) | Thermal Runaway in Lithium-ion Batteries – Improved Test Methodology and Analysis |
| P07 | Katja Lahtinen (Uppsala University, Sweden) | Improving the Performance of Non-Flammable and Non-Fluorinated Electrolytes for Na-ion Batteries |
| P08 | Marie Uth (Danish Graphene, Denmark) | Graphene Additives in LNMO Electrodes |
| P09 | Yaprak Subasi (Uppsala University, Sweden) | Structural and Electrochemical Investigation of Na4Fe3-xMx(PO4)2(P2O7) (M= Mn, Ni, Zn) Cathode for Sodium-Ion Batteries |
| P10 | Jere Leinonen (University of Oulu, Finland) | Effect of Coprecipetation Conditions on Fe0.5Mn0.5CO3 Na-ion Cathode Precursor Properties |
| P11 | Tamara Patranika (Uppsala University, Sweden) | The Interaction and Performance of Boron-Based Self-Healing Binders in Silicon Anodes |
| P12 | Alisa R. Bogdanova (Aalto University, Finland) | Solvent-free Preparation of Binder-free LiNi0.8Mn0.1Co0.1O2 Electrode Enabled via Single-walled Carbon Nanotube Coating |
| P13 | Varsha Joseph Ariyamparambil (Linköping University, Sweden) | Advancing Zinc-ion Batteries: Enhancing cyclic stability and Energy density with Hydrogel Electrolytes and Zinc-lignin systems |
| P14 | Sherif Hegazy (University of Oulu, Finland) | Effect of Fe, B, and Mo Catalysts on the Graphitization and Electrochemical Performance of Biomass-Derived Carbons |
| P15 | Meghdad Hosseinzadegan (Uppsala University, Sweden) | Controlling Sodium Metal Growth by High Overpotential Nucleation in Sodium Metal Batteries |
| P16 | Seyedabolfazl Mousavihashemi (VTT Technical Research Centre of Finland, Finland) | Bio-Waste Derived Materials for Sodium Ion Batteries |
| P17 | Samuel Fretz (SEEL Swedish Electric Transport Laboratory, Sweden) | SEEL Swedish Electric Transport Laboratory – A State-of-the-Art Facility for Electromobility |
| P18 | Anna Kobets (Aalto University, Finland) | Investigating Doping and Calcination Effects on Nickel-Rich Layered Oxide Positive Electrode Material Enhancing the Lithium-Ion Batteries Performance |
| P19 | Johan Jean-Claude Maurice Hamonnet (Norges teknisk-naturvitenskapelige universitet (NTNU), Norway) | P111i4FSI Ionic Liquid as Electrolyte for High-Voltage Lithium-Ion Batteries |
| P20 | Agnes-Matilda Mattson (Uppsala University, Sweden) | Investigating the Electrochemical Intercalation of Lithium into Prussian White using Operando XRD |
| P21 | Artem Faustov (Aalto University, Finland) | Enhancing of High Power Capability and Cycle Life of SWCNT-Incorporated LFP-Cathodes |
| P22 | Chanez Maouche (University of Turku, Finland) | Aza-Quinone Derivatives as Negolytes in Flow Battery Electrochemistry |
| P23 | Marte Skare (Institute for Energy Technology (IFE), Norway) | The Role of Carbon in SiCx Thin Films: A Path to Stable Si Anodes for Li-ion Batteries |
| P24 | Jinsong Hua (Institute for Energy Technology (IFE), Norway) | An Empirical Degradation Model for Li-ion Batteries and its Extension into Second-Life Applications |
| P25 | Carl Erik Lie Foss (Institute for Energy Technology (IFE), Norway) | Optimizing Silicon Anode Composition for Slot Die Coating Using Design of Experiment (DOE) |
| P26 | Julia Wind (Institute for Energy Technology (IFE), Norway) | Cellpy – An Open-Source Python Library for Processing and Analysis of Battery Testing Data |
| P27 | Tove Ericson (Uppsala University, Sweden) | Depth-Resolved Photoelectron Spectroscopy on Cycled Nanostructured SiOx from Microalgae |
| P28 | Isak Drevander (Chalmers University of Technology, Sweden) | Following Hard Carbon Sodiation using Small- and Wide-Angle X-ray Scattering |
| P29 | Sofia Reiner (Chalmers University of Technology, Sweden) | Effects of Voltage Holds on Graphite Electrode Surface Layers and X-ray Photoelectron Spectroscopy Post-Mortem Studies |
| P30 | Paula Keski-Korsu-Piekkari (University of Oulu, Finland) | Effect of Ionic Liquid on Novel Polymer Electrolyte Performance with Different Li-salts Additives |
| P31 | Gian Marco Trippetta (KTH Royal Institute of Technology, Sweden) | Rapid Estimation of the Extent of Lithium Plating via Correlation of NMR and Image Analysis |
| P32 | Yan Lin (University of Oulu, Finland) | Controllable Synthesis and Impact of Oxygen Vacancies on Spinel LiNi0.5Mn1.5O4 Cathodes |
| P33 | Carlos Antonio Rupisan (Aalto University, Finland) | Effect of Galvanic Intermittent Titration Technique Relaxation Time on NMC811 Battery Open Circuit Voltage and Modelling Applications |
| P34 | Princess Stephanie Llanos (Aalto University, Finland) | Cycling Stability of NMC811 Electrode Coated with LiF via Atomic Layer Deposition |
| P35 | Ivy Saha Roy (University of Oulu, Finland) | Towards Sustainable Lithium-Ion Battery Fabrication: Studying a Novel Green Solvent – KJCMPA as a NMP Replacement to Fabricate LFP and NMC-based Battery Cathodes |
| P36 | Rafal Sliz (University of Oulu, Finland) | Solvent Engineering for Sustainable Printing of Multilayer Battery Structures |
| P37 | Esther Poncy Mathew (SINTEF, Norway) | Pre-Lithiation of LIBs using Sacrificial Salt |
| P38 | August Johansson (SINTEF, Norway) | The Battery Modeling Toolbox (BattMo) |
| P39 | Guiomar Hernández (Uppsala University, Sweden) | Environmentally Friendly Electrode Manufacturing with Water-Based and Dry Formulations |
| P40 | Glaydson Simoes dos Reis (Swedish University of Agricultural Sciences, Sweden) | Tree Wastes as Sustainable Precursor for the Synthesis of Macroporous Carbon-Tin (MC/Sn) Anode Composite for Lithium-Ion and Sodium-Ion Batteries |
| P41 | Weicheng Hua (Norges teknisk-naturvitenskapelige universitet (NTNU), Norway) | Evaluation of Different Phosphonium Ionic Liquids for Application with SiO2 Anodes in Li-ion Batteries |
| P42 | Zhichen Ba (Åbo Akademi University, Sweden) | Cyanoethyl Cellulose Reinforced Polymerizable Deep Eutectic Solvent Gel Electrolytes for High-Energy-Density Sodium-Ion Batteries |
| P43 | Amritha P. Sandra (KTH Royal Institute of Technology, Sweden) | Nanochitin Enabled Aqueous Processing of Graphite Electrodes for Greener Lithium-ion Batteries |
| P44 | Lasse Dettmann (Uppsala University, Sweden) | Non-Flammable Liquid Electrolytes for Safer Batteries |
| P45 | Mirna Alhanash (Chalmers University of Technology, Sweden) | Modeling Local Symmetry and Molecular-Level Heterogeneity in Deep Eutectic Electrolytes |
| P46 | Hammad Farooq (Norges teknisk-naturvitenskapelige universitet (NTNU), Norway) | Selective Lithium Recycling from Non-Pyrolyzed NMC111 Black Mass |
| P47 | Amit Sonker (VTT Technical Research Centre of Finland, Finland) | Cellulose Nanocrystals (CNCs) as Binding and Exfoliating Agents for Developing Flexible Composite Films as an Electrode with MoS2 Nanosheets |
| P48 | Mie Engelbrecht Jensen (DTU Energy, Denmark) | Understanding Leaching in Battery Electrodes |
| P49 | Killian Stokes-Rordiguez (SINTEF, Norway) | Investigating pH Effects of Polyacrylic Acid Binders for the Aqueous Processing of LiNi0.5Mn1.5O4 |
| P50 | Morten Johansen (Aarhus University, Denmark) | Structural Effects from Varying the Fe/Mn Ratio in Layered NaFeyMn1-yO2 for Na-ion Batteries |
| P51 | Bettina Pilgaard Andersen (Aarhus University, Denmark) | How much Li can Li3V2(PO4)3 store? |
| P52 | Astrid Holstad Berge (University of Cambridge, United Kingdom) | Understanding the Structure of Al0.36Li5.92La3Zr2O12 using Solid State NMR and Dynamic Nuclear Polarisation |
| P53 | Nikolai Helth Gaukås (SINTEF, Norway) | Laser Sintering as a Tool for Densification of Li6.25Al0.25La3Zr2O12 Films |
| P54 | Xiuyun Zhao (University of Eastern Finland, Finland) | Silicon from Barley Husk for Lithium-Ion Batteries: A Sustainable Approach |
| P55 | Yanqi Xu (Luleå University of Technology, Sweden) | Fluorine-Free Bis(glycolato)borate Anion-Based Salts and Electrolytes: Structures, Properties, and Lithium Compatibility |
| P56 | Gints Kucinskis (University of Latvia, Latvia) | Enhancing NCM111 Cathode Performance with Sustainable Al2O3 Coating via Ethanol-Based Wet-Chemical Synthesis |
| P57 | Esa Hannila (University of Oulu, Finland) | The Effect of Filling Strategies on the Surface Morphology of Valve-Jet Printed Cathodes |
| P58 | Matthijs Holthuijsen (Beyonder, Norway) | Analyzing the SEI on Commercial Battery Electrodes with Enhanced Raman Spectroscopy |
| P59 | Søren Laurs Lopdrup (Aarhus University, Denmark) | Fluoride-Free Water-Processable Electrodes for Sustainable Batteries |
| P60 | Jonathan Fagerström (Institute for Energy Technology (IFE), Norway) | Battery Lifetime Estimations for Freight Train on Partially Electrified Railway Route |
| P61 | Subramani Kaipannan (Norges teknisk-naturvitenskapelige universitet (NTNU), Norway) | Innovative Hard Carbon Materials for High Energy Sodium-Ion Battery Applications |
| P62 | Ali Abo Hamad (Mid Sweden University, Sweden) | Urea Etching of Si Microparticles for Li-Ion Battery Anodes |
| P63 | Nienke Visser (Institute for Energy Technology (IFE), Norway) | All Solid-State Thin Film Li-ion Batteries as Tool to Study Binary Si-based Anodes |
| P64 | Julia Wind (Institute for Energy Technology (IFE), Norway) | A Direct Comparison of a Power and an Energy-Optimized Li-ion Pouch Cell: Characterization, Ageing and Modelling |
| P65 | Helene Lillevestre Langli (Norges teknisk-naturvitenskapelige universitet (NTNU), Norway) | Synthetic Graphites as the Cathode Material for Aluminium-Carbon Batteries |
| P66 | Kristian O. Sylvester-Hvid (DTU Energy, Denmark) | |
| P67 | Weldejewergis Kidanu (Norges teknisk-naturvitenskapelige universitet (NTNU), Norway) | On the Moisture Tolerance of LiFSI-based Lithium-Ion Batteries: A Systematic Study on NMC622/Graphite Cells |
P01: Sewage Sludge Valorization in Energy Storage Systems
Olga Pastushok (Ph.D. student)
Lappeenranta-Lahti University of Technology, Finland
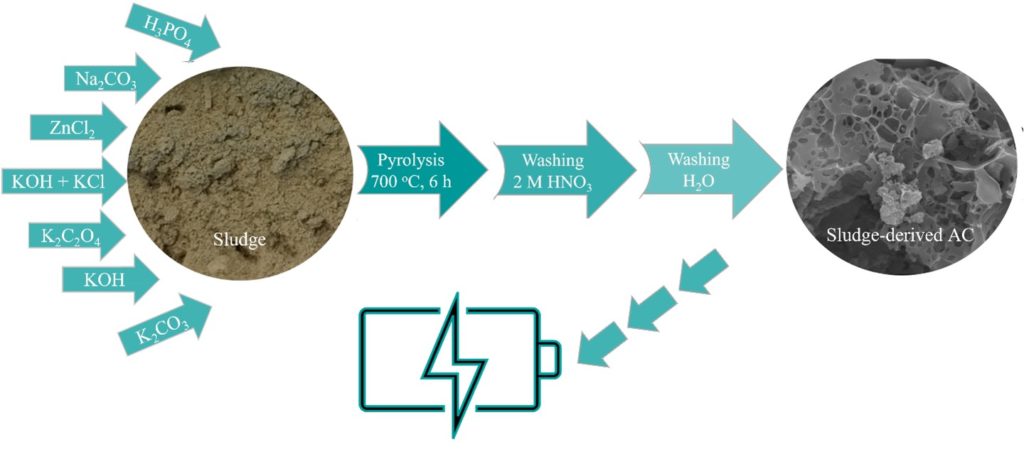
Sewage sludge, a byproduct of the wastewater treatment process, causes a serious environmental concern. Even though, sewage sludge is a source of carbon. Considering global strategies targeting resource recovery and waste reduction, sewage sludge is a promising source for the production of activated carbon. The porous structure of activated carbon provides a large surface area, facilitating increased contact with electrolytes and enhancing the storage capacity for ions.
Admittedly, depending on the activation process and the activator itself, the end product varies in properties. In this work, the most effective chemical activators have been selected to test for the production of sewage sludge-derived activated carbon. Our study aims to investigate the effect of different activators on the electrochemical properties of sewage sludge-derived activated carbon and determine the applicability of the synthesized materials in energy storage systems.
Fig 1. The schematic diagram for the preparation of sewage sludge-derived activated carbon.
P02: Unraveling Boron’s Influence on Enhancing the Performance of Ni-Rich NMC Cathode Materials for Li-Ion Batteries
Lorena Sánchez Moreno (Ph.D. student)
Northvolt AB & Uppsala University, Sweden
Ni-rich NMC (LiNi0.92Co0.04Mn0.04O2) cathode materials in Li-ion batteries (LIBs) offer high capacity, enhancing cell energy density. However, they face challenges like rapid capacity degradation and poor structural stability. Effective stabilization methods include surface (coating) modifications. Boron (B) has emerged as a widely adopted element in surface coatings to improve Ni-rich NMCs’ performance. Yet, the exact mechanisms of B’s positive impact remain unclear due to its complex interactions with NMC. Our study investigates Ni-rich NMC degradation and B’s influence through comprehensive characterization methods before, during, and after cycling. Analytical tools like XRD, XPS, SEM, and electrochemical techniques help examine surface properties. We correlate NMC failure modes with B’s effects, aided by collaborations between Northvolt and Uppsala University, promising advancements in battery research and industry.
P03: Mapping commercial pouch cell electrodes with synchrotron XRD and XRF
David Wragg (Researcher)
Institute for Energy Technology, Norway
Degradation mechanisms in commercial pouch cells (LG JP3) have been studied extensively with electrochemical methods, characterizing state of health and response to various cycling regimes and conditions. To date few links have been made between cycling data for these large cells and the chemical reactions that lead to degradation. Disassembly of JP3 cells cycled under a range of conditions has provided us with many electrode sheets that show visible signs of damage. We have used synchrotron XRD and XRF to map the elemental and crystal structure composition of the electrodes with a spatial resolution of less than 50 microns. Analysis of the XRD data with surface Rietveld methods allows us to extract crystal structure and microstructure parameters for any point in the datasets.
P04: Comparing electrochemical discharge methods for lithium-ion battery recycling
Arjun Muralidharan (Ph.D. student)
Aalto University, Finland
Emerging battery technologies boost energy density, lifespan, and safety. However, the increasing use of lithium-ion batteries (LIBs), coupled with material scarcity and recycling challenges like inefficient processes, and batteries containing various elements, results in low-quality recovered materials. Discharged voltage below 0% state of charge (SOC) is proposed to enable more efficient recycling methods. While aqueous NaCl was suggested, experiments revealed its high corrosiveness during discharge. Experimentation with various inorganic salts in aqueous solutions aimed to achieve low discharge voltage, but water-splitting reactions persisted due to the high voltage of LIBs. Therefore, the organic solvents and ionic liquids in non-aqueous media offer the electrochemical window to maintain stable electrolytes and help mitigate casing corrosion.
P05: Battery Upscaling with Sodium-Ion Chemistry: From Active Material Screening to Pouch Cells
Olli Sorsa (Ph.D / Postdoc)
VTT Technical Research Centre of Finland, Finland
The availability of raw materials is expected to become a limiting factor for the growing market need of lithium-ion batteries. Sodium-ion chemistry is a promising alternative for at least applications where high gravimetrical energy density is not required, e.g. stationary energy storage. With no earlier experience with the sodium-ion chemistry, we started screening different commercially available cathode and anode materials in a sodium-ion battery half-cell. These materials included layered oxides and Prussian blue analogues for cathode and hard carbons produced from different raw materials as anodes. One cathode material and one anode material were chosen for further optimization and upscaling of the electrode coating process. The electrolyte composition is also optimized in full cell configuration for our choice of active electrode materials before upscaling cell assembly to pouch cells. We wish to share the insights we have found during this work in the NordBatt 2024 Conference.
P06: Thermal Runaway in Lithium-ion Batteries – Improved Test Methodology and Analysis
Ola Willstrand (Ph.D. student)
RISE Research Institutes of Sweden & Uppsala University, Sweden
Thermal runaway in Li-ion batteries is associated with rapid heat release rate, large volume of gas production, and violent burning behavior. The focus of battery safety standards is usually to prevent thermal runaway from happening, but that does not eliminate the need for characterization of thermal runaway events. This is an area not well standardized, leading to employment of various test setups, test scenarios, analysis techniques, and interpretation of results.
The main objectives of this work are to investigate the impact of different factors, such as state of charge, thermal runaway triggering methods, ambient atmosphere, and cell size, on the gas generation, gas composition, temperature development, heat generation and mass loss. An important finding is the significant effect from the thermal runaway triggering method, which potentially affects thermal propagation characteristics in battery systems.
P07: Improving the performance of non-flammable and non-fluorinated electrolytes for Na-ion batteries
Katja Lahtinen (Ph.D / Postdoc)
Uppsala University, Sweden
One of the biggest contributors to the battery safety is the electrolyte. Currently, carbonate solutions with fluorine-containing salts are utilized as the most common electrolytes in Na-ion batteries. However, due to the flammability of carbonates and the harmful effects of fluorine, the safety risks and the environmental impact of these electrolyte solutions are considerable. In this work, the focus is on trying to find both non-flammable and non-fluorinated electrolyte combinations to answer these problems. One such an electrolyte is Sodium bis(oxalato)borate (NaBOB) dissolved in triethyl phosphate (TEP) that has shown promising properties, e.g. the ionic conductivity of 5 mS/cm at room temperature. Here the electrochemical properties of NaBOB-TEP are improved with a set of additives and with a goal to understand the formation mechanism of solid electrolyte interphase (SEI) on the hard carbon negative electrode.
P08: Graphene Additives in LNMO Electrodes
Marie Uth (Ph.D. student)
Danish Graphene, Denmark
When adding graphene to spinel LNMO electrodes an increased cycling stability is observed. The lateral size of the graphene sheets and the degree of oxidation affect the performance of the electrode. The presented work shows the optimal graphene concentration for a small flake additive with medium oxidation. The graphene additive can be added as a paste in the slurry mixing step for easy implementation in established electrode preparation procedures. The graphene additive stabilizes the electrochemical transitions under long-term cycling, which results in higher capacity retention.
P09: Structural and Electrochemical Investigation of Na4Fe3-xMx(PO4)2(P2O7) (M= Mn, Ni, Zn) Cathode for Sodium-Ion Batteries
Yaprak Subasi (Ph.D. or Postdoc)
Uppsala University, Sweden
Many efforts have been devoted to the development of high-performance cathode materials for sodium-ion batteries to make them fully competitive to lithium-ion batteries. Na4Fe3(PO4)2(P2O7) (NFPP) is regarded as a promising cathode material due to its non-toxicity, high average working voltage, favorable theoretical capacity, low volume changes as well as structural and thermal stability. However, its practical use is limited because of the formation of impurity phases and low intrinsic electronic conductivity. Various strategies have been developed to enhance conductivity such as nanosizing, carbon coating, metal ion doping. In this study, Na4Fe3-xMx(PO4)2(P2O7) (M: Mn, Ni, or Zn) composites are synthesized to improve capacity and energy density. The structure and morphology of doped NFPP are characterized by X-ray diffraction and scanning electron microscopy techniques. The electrochemical performance is investigated in half-cells via galvanostatic charge-discharge cycling tests.
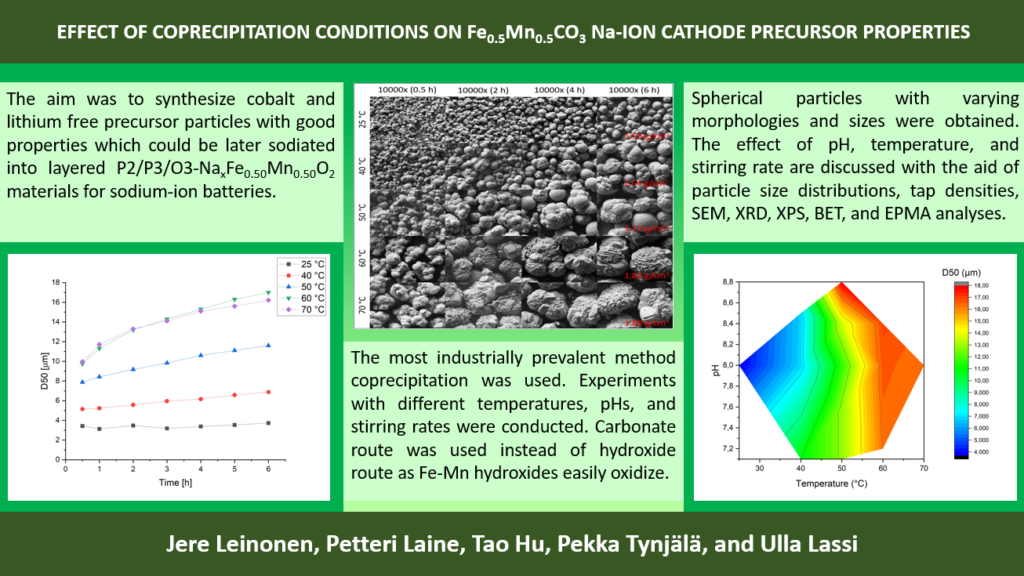
P10: Effect of Coprecipetation Conditions on Fe0.5Mn0.5CO3 Na-ion Cathode Precursor Properties
Jere Leinonen (Ph.D. student)
University of Oulu, Finland
Layered transition metal oxides are an attractive material for Na-ion batteries because of the possibility to use abundant and cheap materials like Fe, Mn, and Na2CO3. However, scientific literature on the effect of coprecipitation parameters on Fe containing cathode precursor materials is lacking. Herein we discuss the effect of pH, temperature, and stirring rate on particle size distribution, tap density, surface area, and morphology for the Fe0.5Mn0.5CO3 precursor. Higher temperature favors the formation of larger particles. Highest tap densities between 1.8-1.9 g/ml were achieved at 60 °C. Particles with usually preferred D50 of ≈10 μm were achieved when temperature was around 40–50 °C. The effect of pH on particle size and tap density is not simple. Even higher stirring rate (>1200 rpm) than what is possible with our experimental setup is preferred to prevent agglomeration. Further research should be done on coprecipitation of Fe1-xMnxCO3 precursors.
P11: The Interaction and Performance of Boron-Based Self-Healing Binders in Silicon Anodes
Tamara Patranika (Ph.D. student)
Uppsala University, Sweden
Silicon is a promising active material for Li-ion batteries, but it faces challenges with volume changes during cycling, limiting the capacity. To address this, we propose a self-healing polymeric binder using dynamic covalent bonds via boronic esters to enhance the cycling stability of Si anodes. Poly(vinyl alcohol) (PVA) cross-linked with 1,4-benzenediboronic acid (BDBA) showed improved performance and less degradation compared to PVA alone. Solid-state NMR revealed changes in the boron environment in the presence of Si, suggesting an interaction between Si and the binder. However, this interaction does not improve the electrochemical performance with styrene-butadiene rubber, which lacks hydroxyl groups, or with carboxymethyl cellulose, despite having hydroxyl groups. Overall, the interaction between BDBA and PVA enhances performance, making it a promising approach for integrating Si in Li-ion anodes.
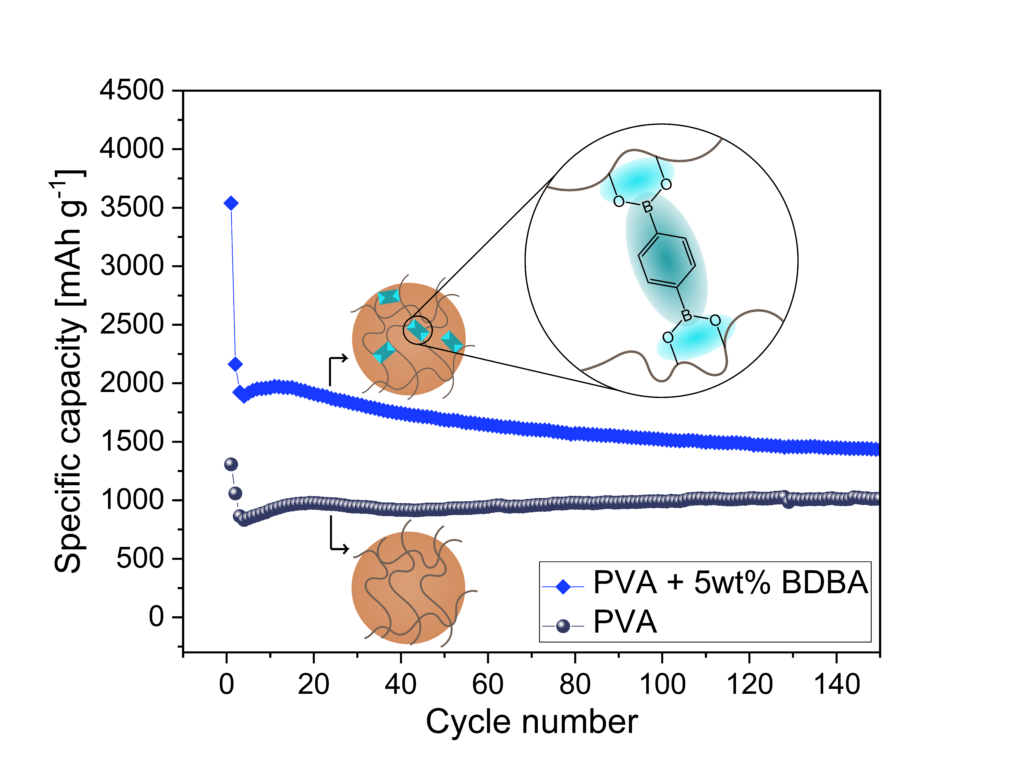
P12: Solvent-free Preparation of Binder-free LiNi0.8Mn0.1Co0.1O2 Electrode Enabled via Single-walled Carbon Nanotube Coating
Alisa R. Bogdanova (Ph.D. student)
Aalto University, Finland
Lithium-ion batteries are a promising type of energy storage devices owing to their optimal combination of performance metrics. However, further advancements require improved battery performance and more efficient manufacturing technologies to address environmental issues. The conventional manufacturing process is energy and resource intensive, involving the dissolution of electrochemically inactive polyvinylidene fluoride binder in the hazardous solvent N-methyl-2-pyrrolidone. Recently, carbon nanotubes have gained interest for dry electrode manufacturing due to their high conductivity and mechanical properties, replacing inactive components. In this work, we employed the concept of in situ gas-phase mixing of single-walled carbon nanotubes (SWCNTs) with aerosolized LiNi0.8Mn0.1Co0.1O2 (NMC811) to obtain binder- and solvent-free electrodes with 99.8 wt% of NMC811. The NMC-SWCNT composite electrodes show high conductivity, rate capability, and enhanced cycling performance.
P13: Advancing Zinc-ion Batteries: Enhancing cyclic stability and Energy density with Hydrogel Electrolytes and Zinc-lignin systems
Varsha Joseph Ariyamparambil (Ph.D. student)
Linköping University, Sweden
Zinc-ion batteries have a huge potential to replace lithium-ion batteries due to their abundant resources and high safety. However, their cyclic stability and energy density need to be improved to compete with lithium-ion batteries. To bridge this gap, research is being done on electrode material, electrolyte, and cell design. Herein, we designed a highly ionic conductive and stable electrolyte, which we accomplished by creating a hydrogel carefully engineered using the water-in-salt electrolyte concept. This eventually led to achieve long-life cycle platting/stripping of Zn. Later, we demonstrated a zinc-Lignin battery having good capacity and cyclic stability.
P14: Effect of Fe, B, and Mo Catalysts on the Graphitization and Electrochemical Performance of Biomass-Derived Carbons
Sherif Hegazy (Ph.D student)
University of Oulu, Finland
Catalytic graphitization of biomass-derived carbon materials presents a promising approach for energy-efficient carbon synthesis. In this study, we investigate the effect of metallic catalysts on the degree of graphitization and electrochemical performance of carbon precursors obtained from hydrothermal carbonization (HTC) of sawdust. We employed Fe, B, and Mo as catalysts at 800°C and 1000°C. The ID/IG ratios obtained from Raman spectroscopy indicated significant graphitization: at 800°C, the ID/IG values were 1.97 for HTC alone, and 0.71, 1.05, and 1.42 for Fe, B, and Mo, respectively. At 1000°C, these values shifted to 1.3 for HTC alone, and 0.69, 0.86, and 0.69 for Fe, B, and Mo, respectively, indicating higher graphitization at the higher temperature. Comprehensive characterization included BET analysis, (SEM), (XRD), This research contributes the understanding of biomass-derived carbon materials’ catalytic graphitization to replace current synthetic and natural graphite in bat
P15: Controlling Sodium Metal Growth by High Overpotential Nucleation in Sodium Metal Batteries
Meghdad Hosseinzadegan (Ph.D. student)
Uppsala University, Sweden
Sodium-ion batteries (SIBs) are the prime alternative to reduce the over-reliance on lithium-ion batteries (LIBs) due to Na and Li similarities, as well as abundancy of Na resources. Finding a high energy density negative electrode is one of the main challenges for SIBs. Although sodium metal has the highest energy density, nonuniform sodium metal growth (e.g., dendrites) can cause short-circuits.
Since the current densities applied during conventional cycling are insufficient to induce a densely populated nucleation event, high overpotential pulses were applied to generate numerous nuclei simultaneously.
Chronopotentiometry, scanning electron microscopy (SEM) as well as operando optical microscopy were performed on symmetrical Na-cells. Results show that a combination of +4 V, and -6 V (both vs. Na+/Na) are most effective to decrease the overpotential during following galvanostatic stripping/ plating. SEM analysis is in agreement showing stabilised and planar Na-metal growth.
P16: Bio-Waste Derived Materials for Sodium Ion Batteries
Seyedabolfazl Mousavihashemi (Ph.D. / Postdoc)
VTT Technical Research Centre of Finland, Finland
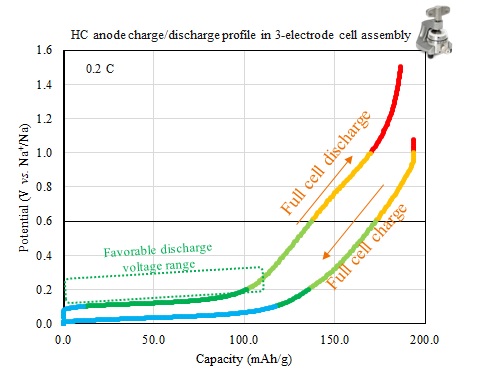
This study presents an electrochemical investigation of sustainable and cost-effective hard carbon (HC) anodes for sodium-ion batteries (SIBs), using bio-waste and side-stream derived materials. SIBs are gaining attention for light electromobility and stationary applications due to their sustainability. Bio-waste resources are ideal HC precursors due to their low cost and abundance. We compared the morphology, microstructure, and electrochemical performance of HCs from different bio sources processed under various conditions. Results show that while the specific capacity of hard carbon anodes is important for material screening, voltage profile analysis is also crucial for selecting materials with the highest energy density. We analyzed the charge and discharge profiles of the HC samples during cycling and selected those with the highest de-sodiation energy density. In conclusion, bio-based HC materials offer a sustainable path for high-performance SIBs, supporting the circular economy
P17: SEEL Swedish Electric Transport Laboratory – A State-of-the-Art Facility for Electromobility
Samuel Fretz (Ph.D. / Postdoc)
SEEL Swedish Electric Transport Laboratory, Sweden
The Swedish Electric Transport Laboratory (SEEL) opened its doors in 2023 and is a state-of-the-art test and research center for electromobility and energy storage located at three sites within Sweden: Gothenburg, Nykvarn, and Borås. Co-owned by Chalmers University of Technology and RISE Research Institutes of Sweden, SEEL provides test facilities to both academia and industry across all levels of electromobility from energy storage research to complete vehicle testing. SEEL’s facilities include a research lab for small, prototype battery cells; commercial battery testing for cell, module, and full pack; a fuel cell lab; an electric vehicle charge lab; driveline testing for electric machines, electric axles, and noise and vibration testing; and light and heavy duty complete vehicle. The Borås site also includes facilities for battery abuse testing. SEEL aims to establish itself as a unique and leading European test center for electromobility.

P18: Investigating Doping and Calcination Effects on Nickel-Rich Layered Oxide Positive Electrode Material Enhancing the Lithium-Ion Batteries Performance
Anna Kobets (Ph.D. student)
Aalto University, Finland
Ni-rich layered oxides like NMC811 are promising positive electrode materials with high specific capacity (200 mAh/g) and energy density, but suffer from rapid capacity decline, structural disorder, and side reactions. Incorporating electrochemically inactive elements like Mg stabilises the layered structure and suppresses Ni ion migration. Calcination conditions during lithiation are crucial for controlling morphology, doping effectiveness, and crystal structure, determining electrochemical behaviour. This work uses three NMC811 precursors with 0, 0.25, and 0.50 at.% Mg content, adding 0.75 at.% Mg during lithiation. The research investigates phase changes via in-situ high-temperature XRD and primary particle growth via SEM at various calcination temperatures to understand the relationship between structural features and electrochemical performance. Combining two types of doping increases specific capacity and stability after 100 cycles at the 3.0-4.6 V vs. Li potential range.
P19: P111i4FSI Ionic Liquid as Electrolyte for High-Voltage Lithium-Ion Batteries
Johan Jean-Claude Maurice Hamonnet
Norges teknisk-naturvitenskapelige universitet (NTNU), Norway
LiNi0.5Mn1.5O4 (LNMO) is a promising high voltage and co-free cathode material for lithium-ion batteries (LiBs) applications. In this work, ionic liquids are being used to develop alternative electrolytes for batteries with enhanced cycling stability and performance.
The electrochemical properties of LNMO/Li half cells with an ionic liquid electrolyte (ILE) composed of 0.79 molal LiFSI in P111i4FSI (figure 1a) are reported. These results are compared to those of the same half-cell with a more conventional 1M LiPF6 in 1:1 ethyl carbonate (EC): diethyl carbonate (DEC) electrolyte (LP40) and at different temperatures. The cells using the P111i4FSI-based electrolytes were more stable, with a discharge capacity still >115 mAh/g at the end of the cycle (figure 1b).
Postmortem analysis showed that the structural integrity of the LNMO particles was significantly damaged after cycling with the LP40 electrolyte and mostly intact with the ILE.
P20: Investigating the Electrochemical Intercalation of Lithium into Prussian White using Operando XRD
Agnes-Matilda Mattson (Ph.D. student)
Uppsala University, Sweden
Most batteries today consist of expensive and scarce metals such as lithium and cobalt. In order to meet the increased demands, other chemistries need to be investigated, for example Sodium-ion batteries (NIBs). An interesting cathode material for NIBs is Prussian White (PW), that is both environmentally friendly and cheap. During use in a battery PW undergoes a phase transition that induces a large volume change. It has been proposed that small ions or neutral guest molecules could mitigate this volume change. In this work the use of lithium to stabilise the framework of PW was investigated using galvanostatic cycling to synthesise the material and Operando XRD to study the phase transition occurring during cycling. The results show that lithium can stabilise the framework by shifting the detrimental phase transition to a higher SOC. As a result, a larger potential window can be utilised without detrimental structural degradation occurring during cycling of the battery.
P21: Enhancing of High Power Capability and Cycle Life of SWCNT-Incorporated LFP-Cathodes
Artem Faustov (Ph.D. student)
Aalto University, Finland
LFP batteries are finding a number of roles in EV and ESS. The drawback of LFP is in moderate specific/volumetric energy densities compared to Co-based cells.
An increase of active material is one of the way to improve these characteristics. SWCNT is bundles of long and flexible nanotubes with high electronic conductivity, can be used in lower amount than carbon black, current conductive additive industrial standard. The author conducted experiments which demonstrate that an usage of SWCNT as conductive additive allows to increase LFP content up to 98% and lead to rate performance and significant cycle life improvement.
Differential capacity done for rate tests shows peak’s shifting. At the same time, no signs of LFP degradation or morphology change is observed for cycled cathodes.
It seems that a mechanical cathode degradation leads to worsening of electrochemical performance. An usage of CNT leads to electrode structure hardening decreasing mechanical degradation during the cycling
P22: Aza-Quinone Derivatives as Negolytes in Flow Battery Electrochemistry
Chanez Maouche (Ph.D. / Postdoc)
University of Turku, Finland
The development of high-density, high-safety, and cost-effective energy storage devices is critical for efficiently utilizing intermittent renewable energy resources. Flow batteries (FBs) are highly promising due to their ability to store large amounts of energy, modularity, and superior safety. FBs consist of two circulating redox couples stored in external electrolyte tanks, storing energy via redox reactions of reduced (negolyte) and oxidized (posolyte) electrolytes. However, the wide-scale use of FBs is limited by cost of redox-active metals. Quinones a promising class of energy storage materials are ongoing research focusing on improving their stability, solubility and performance with a particular emphasis on the creation of stable negolytes. Here, we introduced aza-quinone (AZ) compounds as novel negolytes in FB systems. The electrochemical performance of AZ was evaluated by cyclic voltammetry under various conditions and in FBs at high concentrations to assess their stability.
P23: The Role of Carbon in SiCx Thin Films: A Path to Stable Si Anodes for Li-ion Batteries
Marte Skare (Ph.D. student)
Institute of Energy Technology (IFE), Norway
Binary Si-based compounds like SiCx have demonstrated potential to reduce the capacity fade normally experienced by Si anodes during cycling in Li-ion batteries, but the mechanisms are still unclear. By depositing amorphous SiCx thin films using PECVD, the films stoichiometry and thickness can be readily modified to facilitate a controlled analysis of the materials inherent properties as well as their dependence on the materials composition.
A set of four 70nm thick films has been studied, with four compositions ranging from pure Si to SiC39. XPS confirms formation of SiCx with increasing CH4 to SiH4 ratio during deposition resulting in an increase in Si-C bonds. Grazing-Incidence Pair Distribution Function (GI-PDF) reveal that more carbon also results in reduced long-range order. In electrochemical experiments, increasing carbon content results in lower reversible capacity and initial coulombic efficiency but show signs of differing lithiation mechanisms in dQ/dV analysis.
P24: An Empirical Degradation Model for Li-ion Batteries and its Extension into Second-Life Applications
Jinsong Hua (Researcher)
(Institute for Energy Technology (IFE), Norway)
Lithium-ion batteries suffer from degradation caused by their operational conditions and consequently have a limited lifetime within their first application. These batteries might be available for re-use in a different application, commonly under more moderate usage conditions. The influence of such a change in operating conditions on battery degradation within a second life application is not very well understood.
Here we present the parameterization and subsequent extension of an empirical degradation model that formulates the degradation process as a function of battery operations for both, first- and second life applications. We focus on a commercial 64 Ah NMC-graphite pouch cell. The model parametrization is based on a large dataset containing both calendar and cycle life data of more than 100 cells, covering a large window of temperatures (5-45 °C), C-rates (0.2-1.5 C), SoC windows, and average SoC.
P25: Optimizing Silicon Anode Composition for Slot Die Coating Using Design of Experiment (DOE)
Carl Erik Lie Foss (Researcher)
(Institute for Energy Technology (IFE), Norway)
Slot-die coating is a leading method for industrial-scale anode production due to its high throughput and low operating cost. Transitioning from small lab-scale slurry coating to large-scale production requires optimal slurry composition. Key factors include a low capillary number, achieved through low viscosity, slow foil speed, and high surface tension. However, low viscosity can cause the slurry to run and slow foil speed can reduce production volumes and lead to clogging. The combination of conductive additives, graphite, binder, and solvent content (type and pH) significantly impacts slurry properties. Investigating all possible compositions to optimize these properties is time-consuming. This study aims to minimize the required slurry compositions while obtaining valuable data by using design of experiments (DOE), which is a systematic, efficient method for studying the relationship between multiple input variables and key output variables.
P26: Cellpy – an Open-Source Python Library for Processing and Analysis of Battery Testing Data
Julia Wind (Researcher)
(Institute for Energy Technology (IFE), Norway)
Recent years have witnessed an exponential increase in battery research, driven by the need to develop efficient and sustainable energy storage systems. One of the main tools in battery research is battery cycling experiments, providing insights into the performance, lifetime and quality of the battery. Due to the large variety of battery testing equipment and the resulting multitude of different and often proprietary data formats, combined with the large number of parameters involved, managing and processing battery testing data has often been a difficult and tedious task.
The Python library cellpy assists in solving these problems by
- providing tools to read different data formats,
- converting those into one common data format that also includes relevant battery-specific metadata, and
- providing a data structure equipped with a set of methods that helps the user to easily perform simple and in-depth analyses of both single data sets and collections of data sets.

P27: Depth-Resolved Photoelectron Spectroscopy on Cycled Nanostructured SiOx from Microalgae
Tove Ericson (Ph.D. / Postdoc)
Uppsala University, Sweden
Silicon and SiO2 shows high theoretical storage capacities as anode materials for Li-ion batteries but are challenging to use due to massive volume expansion at Li insertion. One way to counteract this is to use nanostructures. However, the production of such structures often requires complicated methods and hazardous chemicals. A more sustainable approach is to use biosilica produced by organisms, for example diatoms, a unicellular photosynthetic microalgae. The capacity of the naturally produced nano-SiO2 can then be further improved by reducing the material to SiOx. To develop functional batteries based on these species, it is essential to understand the composition, depth profile and stability of the surface layer created when cycling (SEI). Here we use synchrotron radiation with different energies to investigate the SEI layer for this new battery material, cycled with various electrolytes, to enable integration of this sustainably sourced feedstock into battery technology.
P28: Following Hard Carbon Sodiation using Small- and Wide-Angle X-ray Scattering
Isak Drevander (Ph.D. student)
Chalmers University of Technology, Sweden
Hard carbon is regarded as the anode material of choice in sodium-ion batteries. Due to the amorphous structure of the material, the sodiation process in hard carbon anodes is nevertheless still a debated topic in research. In this contribution, a method to study the sodiation process in hard carbon electrodes using small- and wide-angle X-ray scattering is presented. By studying variations in the scattering profile (see fig. 1) at different degrees of sodiation, changes in the hard carbon structure can be deduced. The structural information can then be compared to electrochemical data to infer the underlying storage mechanisms during cell operation. Cells were cycled using two different electrolytes to investigate if varying cell chemistries can affect the sodiation process. Intercalation and pore filling were both found to occur in the hard carbon electrode, but the degree to which the mechanisms contribute to the storage capacity changes between the different electrolytes.
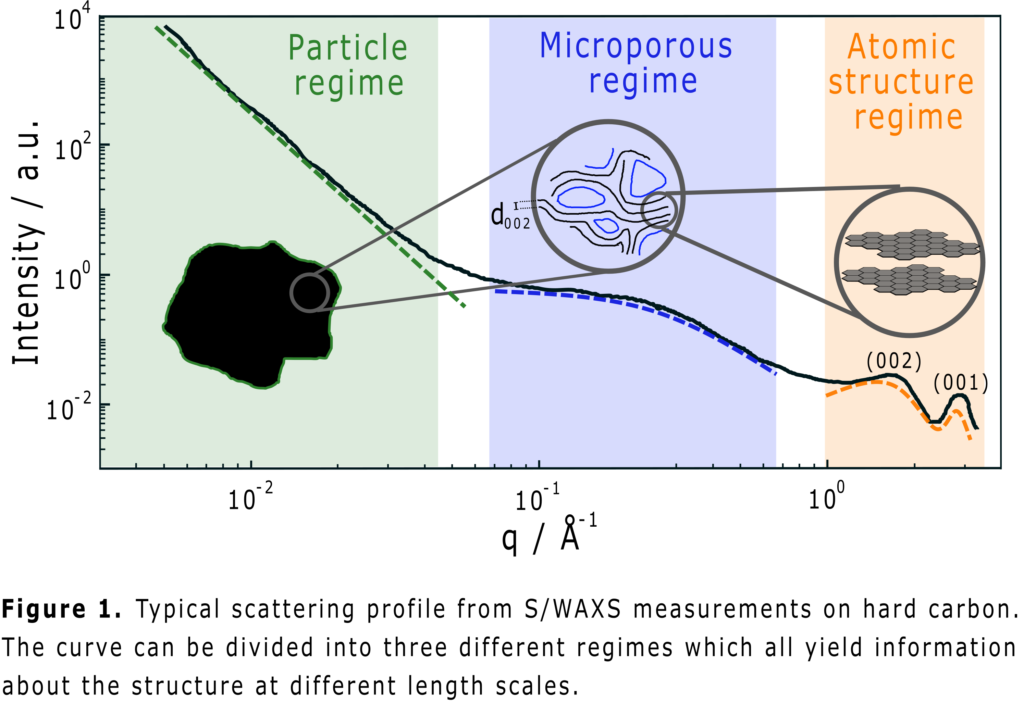
P29: Effects of Voltage Holds on Graphite Electrode Surface Layers and X-ray Photoelectron Spectroscopy Post-Mortem Studies
Sofia Reiner (Ph.D. student)
Chalmers University of Technology, Sweden
In previous studies on lithium-ion batteries, an extended voltage hold during discharge has been shown to improve capacity retention of the cell. In NMC/graphite cells, similar trends have been observed but the mechanisms behind the capacity recovery after voltage holds are not fully understood, and mechanisms connected to the evolution of the solid electrolyte interphase (SEI) cannot be excluded. At the same time, voltage holds are often used in sample preparation for X-ray photoelectron spectroscopy (XPS) studies on electrodes to ensure stable electrode potentials. But what if these voltage holds change the SEI or the general surface composition? Therefore, we study the effect of extended voltage holds on the SEI layer in graphite electrodes with XPS. Furthermore, we present a study into important factors when measuring battery interphases, such as the SEI and areas of plated lithium on graphite. These include the impact of chosen measuring spots, cell type and sample homogeneity.
P30: Effect of Ionic Liquid on Novel Polymer Electrolyte Performance with Different Li-salts Additives
Paula Keski-Korsu-Piekkari (Ph.D. student)
University of Oulu, Finland
In recent studies for safer and efficient batteries, solid state polymer electrolytes (SSPE) have received increasing interest. PVDF is a widely studied binder for SSPE, but it usually needs (inorganic) fillers or ionic liquid (IL) for better ionic conductivity [1, 2]. In this study, we compare PVDF-HFP and commercial Daikin VT475 polymers with MPPyrTDI as IL and two different Li-salts. The ionic conductivity (IC) achieved with PVDF-HFP was 1.11∙10-3 S/cm, whereas with VT475 IC was 1.47∙10-3 S/cm. Various solvents were tested to better understand the membrane formation and its mechanical properties. All membranes were made inside Ar glove box and dried under low vacuum with 300 Torr and 60°C overnight. Membranes of both polymers were colourless, soft, and flexible films. Importantly, higher IL content resulted in poor membrane formation due to difficulties in evaporation. NMP as a solvent provided the best IC with VT475 and LiFTSI salt.
P31: Rapid Estimation of the Extent of Lithium Plating via Correlation of NMR and Image Analysis
Gian Marco Trippetta (Ph.D. student)
KTH Royal Institute of Technology, Sweden
Recent teardown analyses of high-energy Li-ion batteries have revealed a considerable heterogeneity in their degradation, with Li plating as a dominant factor. This presentation will describe a method for rapid estimation of the extent of lithium plating in a high-energy Li-ion battery. Material characterization was performed, using 7Li NMR to quantify Li-plating in different locations. The experimental results were then correlated with a high-definition image analysis, performed by a colour detection algorithm on the scanned electrodes sheets. To produce more accurate results, this protocol was also carried out on the separator, as a significant amount of lithium plating was found adhered to it after tear-down. Finally, results were compared with an electrochemistry-based method called OLSA (OCP-based Local SOH Assessment) to experimentally evaluate how the SOH heterogeneous pattern correlates with lithium plating.
P32: Controllable Synthesis and Impact of Oxygen Vacancies on Spinel LiNi0.5Mn1.5O4 Cathodes
Yan Lin (Ph.D. student)
University of Oulu, Finland
Spinel LiNi0.5Mn1.5O4 (LNMO) is a top candidate for the next generation high-energy and high-power lithium-ion battery cathodes due to its high operating voltage (4.7 V vs. Li+/Li), exceptional specific energy density, impressive rate performance, and cost-effectiveness. The electrochemical properties of LNMO vary significantly between its Ni/Mn ordered P4332 phase and the disordered Fd3m phase, underscoring the importance of achieving specific Ni/Mn arrangements for optimal performance.
In this study, LNMO with varying particle sizes was controllably synthesized using a facile scalable co-precipitation method. The impact of oxygen vacancies on the Ni/Mn arrangement within the LNMO crystal structure was meticulously analyzed using Synchrotron XRD with Rietveld analysis, HRTEM, and XPS. Additionally, in-situ XRD measurement was conducted to delve into the mechanisms of lithium storage within the material, providing deeper insights into its functional dynamics during battery operation.
P33: Effect of Galvanic Intermittent Titration Technique Relaxation Time on NMC811 Battery Open Circuit Voltage and Modelling Applications
Carlos Antonio Rupisan (Ph.D. student)
Aalto University, Finland
Open Circuit Voltage (OCV) behavior of lithium-ion batteries is an important component for modelling applications such as parameter estimation, degradation modelling and state-of-charge estimation, as it represents the static behavior of the battery. One method to obtain this behavior is the Galvanic Intermittent Titration Test (GITT). However, GITT does not have a standard for the relaxation time test parameter, and the amount of time needed to get to open circuit state can be long. This study investigates the effect of shortening the relaxation time for high capacity bare and coated LiNi0.8Mn0.1Co0.1O2 (NMC811) batteries tested at high cutoff potential (4.5 V vs graphite) on battery modelling such as parameter fitting using an equivalent circuit and degradation modelling. Initial results show feasibility to shorten total GITT time by 66% by shortening the relaxation time parameter from a base assumption of 2h to 30min with 2% difference in modelling error for parameter estimation.
P34: Cycling Stability of NMC811 Electrode Coated with LiF via Atomic Layer Deposition
Princess Stephanie Llanos (Ph.D. student)
Aalto University, Finland
Employing high capacity LiNi0.8Mn0.1Co0.1O2 (NMC811) as a cathode material can help deliver high-energy-density lithium-ion batteries. However, a high voltage operation can lead to irreversible side reactions at the electrode-electrolyte interface which result in drastic capacity fade. In this work, a stable nanoscale lithium fluoride (LiF) coating is deposited on the NMC811 electrode via atomic layer deposition to address the interfacial instability issues. The LiF coating serves as an artificial cathode electrolyte interface (CEI) and prevents the direct contact between NMC811 and electrolyte to suppress harmful parasitic reactions during cycling. LiF-NMC811 delivers higher capacity retention than uncoated NMC811 after 100 cycles at a high cutoff potential for half-cell (3.0-4.6 V vs Li/Li+) and full-cell (2.8-4.5 V vs graphite) configurations. As LiF is a main component in the CEI, the study highlights the impact of CEI in NMC-based cathodes.
P35: Towards Sustainable Lithium-Ion Battery Fabrication: Studying a Novel Green Solvent – KJCMPA as a NMP Replacement to Fabricate LFP and NMC-based Battery Cathodes
Ivy Saha Roy (Ph.D. student)
University of Oulu, Finland
N-methyl-2-pyrrolidone (NMP), commonly used in lithium battery cathode processing, necessitates a safer, greener alternative due to its high toxicity and regulatory restrictions. Our study explores the use of KJCMPA100 (3-methoxy-N,N-dimethylpropanamide), a novel, eco-friendly solvent, as a sustainable alternative to NMP in the fabrication of both LFP and NMC811 lithium-ion battery cathodes. KJCMPA100, when compared to a green solvent Cyrene (Figure 1a), exhibits superior properties such as reduced boiling point, viscosity, and surface tension, indicating enhanced wettability and adhesion[1]. Furthermore, the integration of HSV1810, a novel high molecular weight binder, results in improved conductivity compared to PVDF [1] [2]. The research also investigates the capabilities of two different printing methods, with a particular emphasis on the efficacy of spray printing (Figure 1b-f) in overcoming the thickness limitations inherent in screen printing [3].
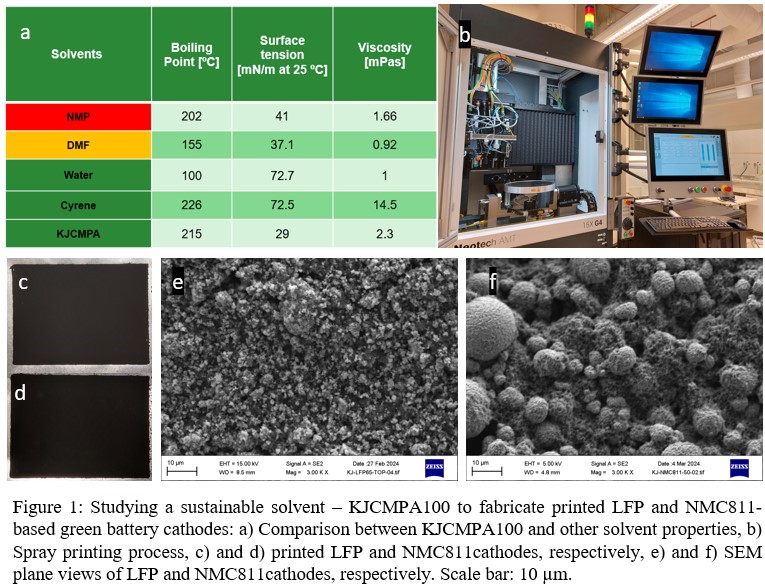
P36: Solvent Engineering for Sustainable Printing of Multilayer Battery Structures
Rafal Sliz (Prof.)
University of Oulu, Finland
As the use of NMP as a solvent becomes increasingly restricted, significant efforts are being directed towards identifying sustainable, non-toxic alternatives that provide comparable dissolving properties. While Cyrene, KJCMPA, PolarClean, etc., show promising outcomes, they present challenges in processing, and the resulting battery components often do not match the performance of those produced with NMP. Additionally, when creating multilayer battery architectures through additive manufacturing, the interfacial interactions are crucial.
This work introduces an innovative multisolvent approach that utilizes a minimal amount of NMP in a pre-dissolving stage, followed by the use of a sustainable solvent as the primary medium for slurry creation and further processing. This solvent engineering strategy enables the formulation of slurries with tailored properties, facilitating the controlled deposition of battery multilayer structures with highly adhesive interfaces.
P37: Pre-Lithiation of LIBs using Sacrificial Salt
Esther Poncy Mathew (Ph.D. student)
SINTEF, Norway
LNMO (Lithium Nickel Manganese Oxide), a high-voltage spinel compound, is a promising cathode material to power future electric vehicles when coupled with silicon-based anodes. Pre-lithiation strategies using sacrificial salts are studied to improve its cycling performance. Lithium oxalate, a well-studied pre-lithiation salt which is cheap and compatible with the standard cathode processing protocol is investigated. Different methods to overcome the kinetic hurdles of salt decomposition is studied to enable the full utilization of the pre-lithiation salt. The salt incorporation into the cathode material is optimised and the cycling behaviour of lithium oxalate enriched LNMO is analysed.
P38: The Battery Modeling Toolbox (BattMo)
August Johansson (Researcher)
SINTEF, Norway
BattMo [1] is an open-source framework for simulating electrochemical-thermal devices jointly developed by electrochemists, physicists, and applied mathematicians. It is primarily developed for Li-ion batteries described by PXD (X=2,3,4) Doyle-Fuller-Newman models, and has been tested and verified on various electrode materials, including SiGr, NMC, LFP, and LNMO, as well as standard formats such as coin, pouch, and cylindrical cells. Its modular design allows for application to other systems such as magnesium batteries and electrolysers. BattMo is developed in MATLAB and also has a fast Julia version called BattMo.jl [2] in development. This contribution presents the key features of the software, especially its parameterization capabilities, how simulations can be fully described in JSON format, and its GUI [3]. Documentation is available at [4].
- www.github.com/BattMoTeam/BattMo
- www.github.com/BattMoTeam/BattMo.jl
- app.batterymodel.com
- www.battmoteam.github.io/BattMo-doc
P39: Environmentally Friendly Electrode Manufacturing with Water-Based and Dry Formulations
Guiomar Hernández
Uppsala University, Sweden
With the increasing battery production and demand, not only the choice of materials should be considered for sustainability purposes but also the processes involved. These should be more environmentally friendly, energy efficient and avoid the use of toxic components. The Horizon Europe project NoVOC “Eliminating VOC from battery manufacturing through dry or wet processing” aims to develop more sustainable electrode manufacturing processes for NMC811 and silicon-graphite composites including both aqueous-based and dry formulations. In addition, the project aims to reduce or omit PFAS-based compounds with fluorine-free alternatives. Herein, the most recent results of the project will be presented.
P40: Tree Wastes as Sustainable Precursor for the Synthesis of Macroporous Carbon-Tin (MC/Sn) Anode Composite for Lithium-Ion and Sodium-Ion Batteries
Glaydson Simoes dos Reis (Researcher)
Swedish University of Agricultural Sciences, Sweden
A biomass-based carbon tin oxide (SnO2) composite anode (BC/Sn) was prepared and compared to its pure carbon (BC) form as a candidate for lithium-ion (LIBs) and sodium-ion (NIBs) batteries. The characterization data proved that the SnO2 had a remarkable impact on MC/Sn physicochemical properties, favoring the MC/Sn anode electrochemical performance compared to BC in LIBs and NIBs. For LIBs, MC/Sn anode delivered a specific capacity of 319 mAhg−1 at the end of 120 cycles, while pure carbon delivered only 93.2 mAhg−1 at the end of 120 cycles (at 1C). Moreover, when MC/Sn was subjected to a cycling performance at 0.1C, it exhibited a capacity of 453 mAhg−1 at the end of 120 cycles. When both samples were tested in NIBs, MC/Sn exhibited better cycling stability giving a higher capacity during all cycles compared to pure carbon anode, delivering a capacity of 204 mAhg-1 at the end of 162 cycles, while pure carbon delivered a capacity of 165 mAhg-1 at 100mAg-1.
P41: Evaluation of Different Phosphonium Ionic Liquids for Application with SiO2 Anodes in Li-ion Batteries
Weicheng Hua (Ph.D. student)
Norges teknisk-naturvitenskapelige universitet (NTNU), Norway
Ionic liquids (ILs) are a safer electrolyte alternative over conventional electrolytes for Li-ion batteries. Specifically, phosphonium based ILs are investigated due to their large electrochemical stability window, high lithium salt solubility and stable SEI formation. Three different phosphonium ILs (P1112, P1113, P111i4) for application with diatom-SiO2 anodes are reported. The physio-chemical properties were first studied by thermal (TGA) and thermodynamic (DSC) analysis, and an increase in thermal stability with the alkyl group length is found. Cyclic voltammetry results showed that the phosphonium IL electrolytes are stable between within a range between -0.1 V and 4.5 V vs. Li+/Li. To study Li metal stability at the anode side, Li symmetric cells were aged to give insight on the stability of the Li/IL interphase layer. Finally, the ILs outperform conventional liquid electrolyte in galvanostatic tests done at both room temperature and 45°C and P1113 showed the best performance.
P42: Cyanoethyl Cellulose Reinforced Polymerizable Deep Eutectic Solvent Gel Electrolytes for High-Energy-Density Sodium-Ion Batteries
Zhichen Ba (Ph.D. student)
Åbo Akademi University, Finland
Solid-state sodium-ion batteries using gel electrolyte with improved safety and high theoretical capacity are considered as the next generation of high-energy-density batteries. However, the inferior interfacial stability and sharp drop in conductivity at low temperatures hinder their practical applications. In this study, a NaTFSI/hydroxyethyl methacrylate polymerizable deep eutectic solvent is used to construct a low-temperature-resistant gel electrolyte by introducing cyanoethyl cellulose network, which aims to promote the dissociation of salts and thus improving the ionic conductivity remarkably (0.66 mS cm-1 at 25 oC). The interface is reinforced after initiating polymerization under UV to prevent sodium dendrites. Furthermore, an extremely wide high thermal stability are proved by low freezing point at -70 oC and non-decomposition temperature at 300 oC. This study provides a broader promise towards safe, low-cost, and high-performance electrolytes in sodium-ion batteries.
P43: Nanochitin Enabled Aqueous Processing of Graphite Electrodes for Greener Lithium-ion Batteries
Amritha P. Sandra (Ph.D. student)
KTH Royal Institute of Technology, Sweden
The demand for lithium-ion batteries is growing due to the expanding electric vehicle and portable electronics markets. Sustainable and safe battery technologies are essential. Binders, though low in volume, are crucial as they interconnect the active material and current collector, influencing electrode processing. PVDF is commonly used but requires hazardous organic solvents like NMP, which are not eco-friendly. Hence, large-scale aqueous-based processing is crucial to reduce LIB production’s environmental impact. We present colloidal nanostructured chitins from fisheries waste as a fluorine-free, sustainable binder for LIB electrodes. This nanochitin enables aqueous processing with minimal binder content and no need for extra surfactants to disperse hydrophobic materials in water. The electrochemical performance of Gr | Li half-cells was compared with PVDF-based electrodes. Online electrochemical mass spectrometry was used to study binders’ impact on parasitic gas evolution in LIBs.
P44: Non-Flammable Liquid Electrolytes for Safer Batteries
Lasse Dettmann (Ph.D. student)
Uppsala University, Sweden
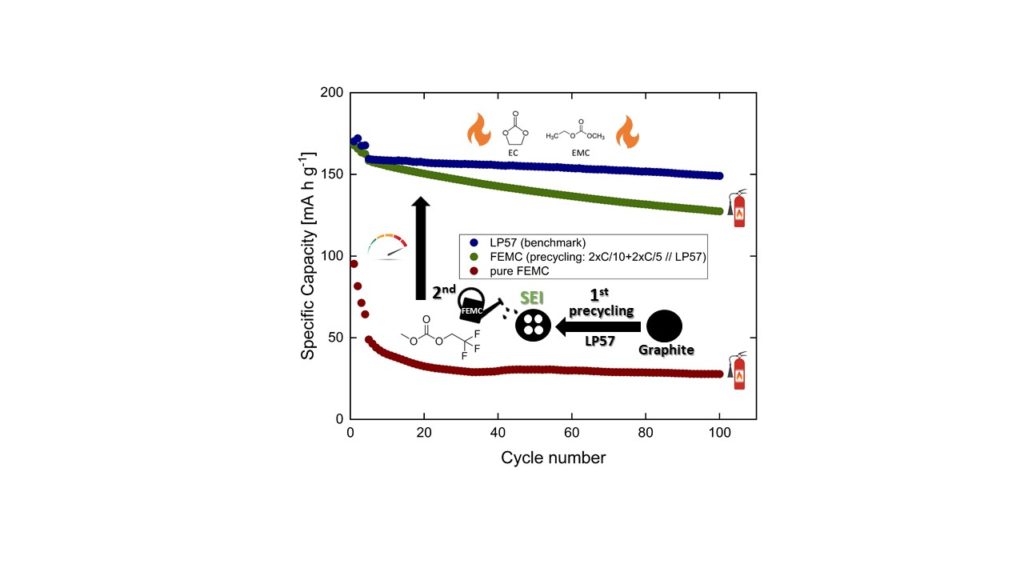
Lithium-ion batteries (LIBs) are widely used across many sectors, including electric bikes and electric vehicles, where safety is crucial, but their conventional liquid electrolytes are highly flammable. Next-generation non-flammable electrolytes like FEMC (2,2,2-trifluoroethyl carbonate) are of great interest for improving safety but often fail to form a passivating solid electrolyte interphase (SEI) on the negative electrode. This results in performance that falls short of commercially available solutions. One promising strategy to address this is pre-passivating the electrode using a state-of-the-art electrolyte such as LP57 (EC/EMC 3:7) before switching to the safer FEMC for long-term operation. We combined detailed electrochemical measurements from our home laboratory with synchrotron photoelectron spectroscopy depth profiling to find out why the pre-passivated electrode protects the electrolyte from further decomposition and to determine the optimum conditions for this step.
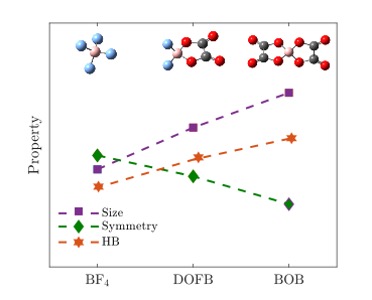
P45: Modeling Local Symmetry and Molecular-Level Heterogeneity in Deep Eutectic Electrolytes
Mirna Alhanash (Ph.D. student)
Chalmers University of Technology, Sweden
Deep eutectic electrolytes (DEEs) have emerged as a promising alternative to conventional liquid electrolytes for lithium-ion batteries (LIBs) and lithium metal batteries (LMBs). The relationship between the molecular-level structure and the local structure and symmetry of DEE components is not yet well understood. Thus, a deeper understanding of how the anion symmetry and size influence the hydrogen bonding (HB), which affects the structural organization and macroscopic behaviour is needed.We have used molecular dynamics (MD) simulations to investigate three basic DEEs consisting of N-methyl-acetamide (NMA) and one of the three common and simple lithium salts (LiBF4, LiDFOB, and LiBOB). Our findings indicate that lower symmetry and larger anions result in greater molecular-level heterogeneity, with this local coordination variance being closely linked to the HB interactions. Overall, the anion size correlates with HB positively while the anion symmetry correlates negatively (Fig. 1).
P46: Selective Lithium Recycling from Non-Pyrolyzed NMC111 Black Mass
Hammad Farooq (Ph.D. student)
Norges teknisk-naturvitenskapelige universitet (NTNU), Norway
The growing demand for lithium-ion batteries (LIBs) has significantly strained the primary resources of lithium (Li) and graphite, necessitating efficient recycling of waste LIBs (black mass). Herein, we show that Li can be selectively recycled from a non-pyrolyzed NMC111-type black mass by leaching it with water. The results showed that pH is the dominant factor during Li leaching, and the optimized parameters were 80 °C, 30 min reaction time, and pH 10. It was observed that CLi in the leachate ranges from 100 – 500 mg/L, and to extract this Li, several ion exchange resins were used to study the uptake capacity, selectivity, and the effect of impurities. The results from batch setup showed that Li was efficiently extracted, and later up-concentrated by a factor of 12 – 14. The process is optimized for continuous operation and evaluated with many Li-waters e.g., artificial solution, leachates and flotation water post-graphite recovery thus enabling efficient and scalable Li extraction.
P47: Cellulose Nanocrystals (CNCs) as Binding and Exfoliating Agents for Developing Flexible Composite Films as an Electrode with MoS2 Nanosheets
Amit Sonker
VTT Technical Research Centre of Finland, Finland
The study presents an efficient method for exfoliating MoS2 in water, using it as an active electrode material, achieving a 90% Coulombic efficiency in zinc battery half-cells. The electrodes consisted of exfoliated MoS2, nanocrystalline cellulose, and carbon nanotubes. The exfoliation process, conducted by sonication with sulfated cellulose nanocrystals (CNC) for 4 hours, was validated by Raman spectroscopy and TEM, which confirmed a blue shift in the MoS2 bands indicative of CNC-induced lattice strain. The MoS2 suspension remained stable, showing no signs of precipitation after two months of standing. The zeta potentials were measured at −45 mV for sodium sulfated CNC–MoS2 and −35 mV for sulfated CNC–MoS2. Sodium sulfated CNCs exhibited micelle formation at a critical aggregation concentration of 1.1 wt%, effectively exfoliating MoS2 at concentrations lower than those required for synthetic surfactants such as SDS (Sodium dodecyl sulfate) and CTAB (Cetyltrimethylammonium bromide.
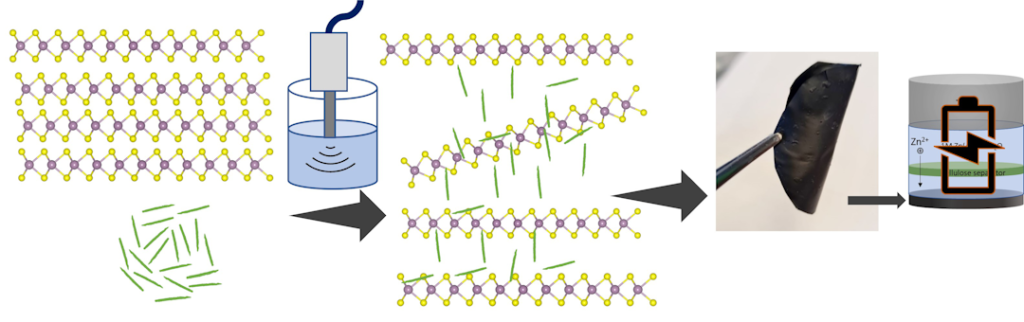
P48: Understanding Leaching in Battery Electrodes
Mie Engelbrecht Jensen (Ph.D. student)
DTU Energy, Denmark
Large quantities of battery waste from electric vehicles will soon build up. Improved recycling technologies are needed. Hydrometallurgical recycling is a more sustainable alternative to pyrometallurgical recycling due to the lower energy consumption. The climate impact of hydrometallurgical recycling depends on chemical consumption as the technology is based on leaching. The initial steps of the transition metal (TM) dissolution at the electrode leachate interface are not understood. Theoretically, a reductant is required for metal dissolution to occur. Experimentally, it is known that up to 40% of the TMs in NMC can be recovered in acid without a reductant. Computational methods can be used to model electrode leachate interfaces and determine the initial dissolution steps. Here we apply density functional theory calculations to study leaching processes. Our findings will provide fundamental understanding of battery leaching processes and guide the development of new processes.
P49: Investigating pH Effects of Polyacrylic Acid Binders for the Aqueous Processing of LiNi0.5Mn1.5O4
Killian Stokes-Rordiguez
SINTEF, Norway
Aqueous processing of transition metal oxide cathodes is highly desirable for reducing processing costs and improving sustainability. If combined with a cobalt-free cathode, such as spinel LNMO, this strategy can significantly reduce the environmental impact of LIBs while providing a high energy cathode for electric vehicle applications. However, aqueous processing of LNMO remains challenging due to pH increases and current collector corrosion. This study investigates the stability of LNMO in water processible polyacrylic acid (PAA) as a function of pH. The pH of PAA was adjusted using Na or Li salts to form NaPAA and LiPAA with pH values ranging from 5 to 8. The binders were evaluated on their pH buffering capabilities during aqueous processing of LNMO electrodes. ICP-MS was used to characterize the amount of transition metal leaching from LNMO at the different binder pHs. The electrochemical performance of the prepared electrodes was evaluated and benchmarked against PVDF cathodes.
P50: Structural Effects from Varying the Fe/Mn Ratio in Layered NaFeyMn1-yO2 for Na-ion Batteries
Morten Johansen (Ph.D. / Postdoc)
Aarhus University, Denmark
Most Li-ion batteries rely on chemistries inclosing toxic and scarce elements such as Co, Ni and Li. Thus, developing novel and sustainable alternative technologies based on abundant elements such as Na, Mn and Fe is of high priority [1, 2]. During recent years sodium ion batteries have greatly improved and become a promising substitute for the current Li-ion technology. Herein the layered transition metal oxides are promising class of electrode material due to their high capacity. However, we still lack to identify materials with truly sustainable elemental compositions, which provides stable capacities with repeated dis-/charge of the battery. Materials based on iron and manganese, NaFeyMn1-yO2 are of high interest due to the high abundance of these elements and reports have shown capacities exceeding 160 mAh/g [3-5]. The layered transition metal oxides are typically obtained in one of two polymorphs denoted as the P2- or O3-phase. The O3-phase is interesting due to a higher initial sodium loading, i.e. higher capacity, compared to P2-analogoues.
In this study, we investigate how the structural phase transitions are affected by varying the Fe:Mn ratio in O3-NaFeyMn1-yO2 (y = 0.5, 0.6, 0.7 and 0.8). The materials with high Fe-content, NaFe0.7Mn0.3O2 and NaFe0.8Mn0.2O2, transform via second order phase transitions into an unknown O3-like phase upon Na intercalation, while samples with low Fe content, NaFe0.5Mn0.5O2 and NaFe0.6Mn0.4O2, go through multiple phase transitions involving (figure 1). Interesting, the first and second charge differs in NaFe0.5Mn0.5O2, where an unidentified “X-phase” forms due to severe stacking faults during the second cycle. Our analysis of the X-phase shows that it bears a closer resemblance to an O3-state than a P3-state, suggesting a higher probability of finding an octahedral layer compared to prismatic Na coordination.
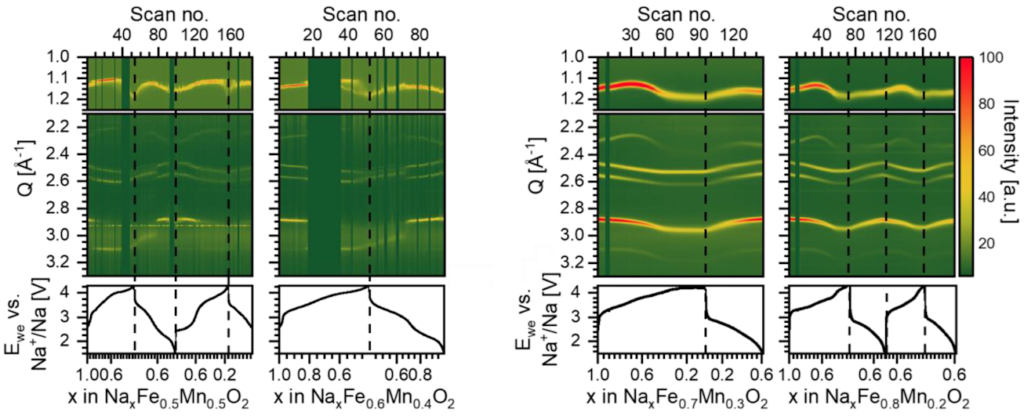
P51: How much Li can Li3V2(PO4)3 store?
Bettina Pilgaard Andersen (Ph.D. / Postdoc)
Aarhus University, Denmark
Rechargeable batteries are widely used in today’s society. To keep up with the demand and be able to meet the requirements of present and future technologies, it is necessary to discover and develop new electrode materials. However, it is also crucial to be able to take full advantage of and improve already existing materials and understand the echanisms taking place during operation of the battery cell. One of these materials is α-Li3V2(PO4)3 which, as a positive electrode, has received a lot of attention due to its high theoretical capacity, good capacity retention, and relatively high ionic conductivity. However, recent studies have shown that it is not only possible to extract the existing three Li-ions in the structure [1], but it should be possible to insert additional Li-ions as well [2-5]. To exploit the full capacity range of the material it is necessary to determine the more exact Li-storage capabilities as earlier studies do not agree about the number of Li-ions possible to store, suggesting between 5 and 9 Li-ions [2-5]. Furthermore, it is necessary to investigate the structural transitions taking place as previous studies have mainly used arguments based on electrochemical measurements [2-5].
In this study, α-Li3V2(PO4)3 is used in a half cell against Li metal to study the structural evolution at both deep charge and discharge, utilizing techniques as operando powder X-ray diffraction and Rietveld refinements, ex-situ total scattering and pair distribution function analysis, ex-situ X-ray absorption near edge spectroscopy, and void space analysis. Thereby, valuable information about the structural evolution, phase transitions, and evolution of the oxidation state of vanadium is obtained, giving more insight into the capabilities of α-Li3V2(PO4)3 as an electrode material.
P52: Understanding the Structure of Al0.36Li5.92La3Zr2O12 using Solid State NMR and Dynamic Nuclear Polarisation
Astrid Holstad Berge (Ph.D. student)
University of Cambridge, United Kingdom
Current battery research focuses on improving and overcoming the remaining challenges facing batteries, namely increasing their longevity, energy density and safety. One strategy is to substitute the lithium conducting liquid electrolyte with a solid electrolyte. Solid electrolyte-based Li-ion batteries can enable energy storage devices with high energy and power densities whilst also being safer than liquid electrolytes.1
Since it was first synthesised in 2007, Li7La3Zr2O12 (LLZO) has received attention as a promising candidate for solid electrolytes due to its high conductivity and stability against Li metal.2 LLZO is a tetragonal Li+ conductor which upon doping with a cation forms a cubic structure. The cubic lattice has better connectivity of Li+ sites and a higher number of Li+ vacancies, increasing the Li-ion conductivity by two orders of magnitude.3 Despite the dopant atom’s key influence on the conductivity, there is debate in the field regarding the atomic positions of the dopant in the solid electrolyte.
In this study, Al3+ doped LLZO (Al0.36Li5.92La3Zr2O12) was synthesised and the 27Al Nuclear Magnetic Resonace (NMR) signals were recorded showing three aluminium environments in LLZO. Using a Double Quantum Single Quantum NMR experiment, these peaks were identified to be Al doped in a tetragonal (24d) site in LLZO and Al in two impurities, LiAlO2 and LaAlO3. Furthermore, Ga3+ doped LLZO (Ga0.2Li6.3La3Zr2O12) was synthesisted and the two 71Ga NMR resonances were identified as Ga3+ in LLZO and LiGaO2.4.
To further investigate LLZO, a mixture of endogenous and exogenous Dynamic Nuclear Polarisation (DNP) NMR was performed. Using a combination of direct 27Al DNP and a 7Li – 27Al D-HMQC DNP experiment, the environment of Al in LLZO and the degradation near LiAlO2 and LaAlO3 were explored.
- J. Janek, W. G. Zeier, Nat Energy 2016, 1, 16141
- R. Murugan, V. Thangadurai, W. Weppner, Angew. Chem. Int. Ed. 2007, 46 (41), 7778-7781
- J. Awaka, A. Takashima, K. Kataoka, N. Kijima, Y. Idemoto, J. Akimoto, Chem Lett 2011, 40 (1), 60–62
- S. Vema, A. H. Berge, S. Nagendran, C. P. Grey, Chemistry of Materials 2023, 35 (22), 9632-9646
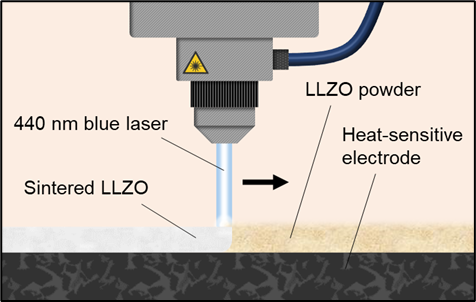
P53: Laser Sintering as a Tool for Densification of Li6.25Al0.25La3Zr2O12 Films
Nikolai Helth Gaukås (Researcher)
SINTEF, Norway
Solid-state batteries with oxide electrolytes are considered as a promising next-generation energy storage technology due to their high energy density, improved safety, and long cycle life. However, developing reliable and scalable techniques for integration of a thin solid electrolyte with heat-sensitive electrode components remains a fundamental challenge in the field. In this work we explore laser sintering as a tool for local consolidation/sintering of ceramics down to the micrometer range, and how this technology can be utilized for in-line processing of battery components. As a test-case we use a garnet-type ceramic, Al-doped LLZO (Li6.25Al0.25La3Zr2O12), and we investigate how laser sintering affects the density and phase composition of LLZO films and bulk structures compared to regular furnace sintering. Finally, we demonstrate how powder morphology and light absorption characteristics play a crucial role in the laser sintering process of LLZO.
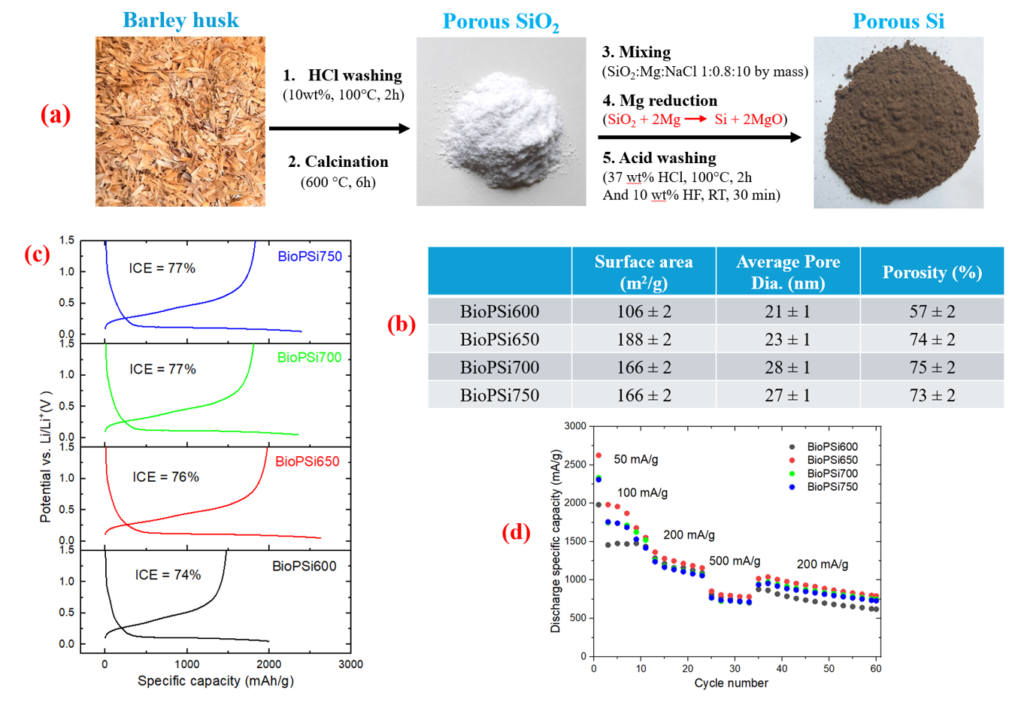
P54: Silicon from Barley Husk for Lithium-Ion Batteries: A Sustainable Approach
Xiuyun Zhao (Researcher)
University of Eastern Finland, Finland
The need for more efficient and sustainable energy storage systems has intensified the search for advanced materials in lithium-ion batteries. Silicon (Si), with its high theoretical capacity of around 3579 mAh/g, has emerged as a leading candidate for next-generation anodes. However, its widespread application is challenged by severe volume expansion during charge/discharge cycles, leading to rapid capacity fading and structural failure [1]. The porous structure is a promising solution to these challenges by accommodating the volume change during battery cycling and improving electrolyte access [2,3]. This study presents the synthesizing process of porous silicon from barley husk, an abundant and renewable agricultural waste [4], and applying the obtained Si in lithium-ion batteries. Barley husks were processed through acid leaching, calcination, and magnesiothermic reduction to transform silica (SiO₂) into porous silicon (Figure 1a). The nitrogen sorption technique was adopted to analyze its porous structure and assess its surface area (Figure 1b). The results indicated that these pore parameters are controlled by the reduction temperature. Electrochemical testing of the porous silicon anodes revealed that the optimized Si was obtained at 650°C, which delivered an initial specific discharge capacity of 2624 mAh/gSi and showed better cycling stability than other samples. In the next step, pre-lithiation will be applied to improve the initial coulombic efficiency. Also, the silicon surface will be stabilized with thermal carbonization to improve the cycling stability of the BioPSi electrode.
The use of barley husk provides an eco-friendly and cost-effective silicon source but also imparts unique porous characteristics that benefit lithium-ion battery applications. This work demonstrates the potential of converting agricultural waste into high-value materials, contributing to sustainable battery technology and waste valorization.
Acknowledgment
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 958174 (M-ERA.NET 3) and is supported by the Academy of Finland project number 325495.
References
[1] X. Zhao, V. -P. Lehto, Nanotechnology, 32 (2021) 042002.
[2] X. Zhao, N. Kalidas, V.-P. Lehto, J. Power Sources, 529 (2022) 231269.
[3] M. Pillai, X. Zhao, N. Kalidas, K. Tamarov, V.-P. Lehto, Microporous Mesoporous Mater. 367 (2024) 113004.
[4] N. Kalidas, J. Riikonen, W. Xu, K. Lahtinen, T. Kallio, V. P. Lehto, Mater. Chem. Phys., 245 (2020) 122736.
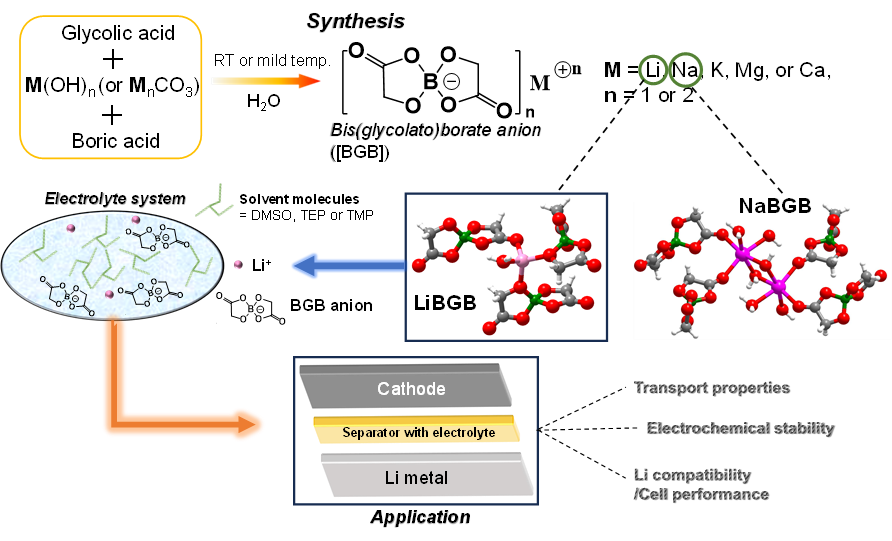
P55: Fluorine-Free Bis(glycolato)borate Anion-Based Salts and Electrolytes: Structures, Properties, and Lithium Compatibility
Yanqi Xu (Ph.D. student)
Luleå University of Technology, Sweden
A family of bis(glycolato)borate (BGB) anion-based salts, also comprising Li+, Na+, K+, Mg2+ and Ca2+ cations, has been synthesized and characterized. Several new fluorine-free electrolytes based on LiBGB and organic solvents such as dimethyl sulfoxide (DMSO), triethyl phosphate (TEP), and trimethyl phosphate (TMP) have been created and their transport properties, electrochemical stability and lithium compatibility examined. The ionic conductivities of the 1 M LiBGB-TEP and 1 M LiBGB-TMP electrolytes are ca. 2-3 times lower than the 1 M LiBGB-DMSO electrolytes, and as compared to the state-of-the-art LP40 they display fewer ionic conductivities, but the formers’ redox stability on aluminum (Al) and glassy carbon electrodes are much better. Concentrated (>1 M) LiBGB-DMSO electrolytes display enhanced redox stability as well as higher ionic conductivities than the LiBGB-TEP and LiBGB-TMP electrolytes, but worse Al passivation. Among the electrolytes, 1 M LiBGB-TMP achieves the best rate performance and long-term stability for Li plating-stripping. Overall, this study introduces a family of versatile fluorine-free orthoborate salts and electrolytes for mono- and divalent batteries, as well as it provides a fundamental understanding of their transport and electrochemical properties, aiming towards battery applications.
P56: Enhancing NCM111 Cathode Performance with Sustainable Al2O3 Coating via Ethanol-Based Wet-Chemical Synthesis
Gints Kucinskis (Researcher)
University of Latvia, Latvia
LiNixCoyMn1-x-yO2 (NCM) cathodes, especially those with high nickel content, are critical for advancing the energy density of lithium-ion batteries yet face significant challenges in maintaining good cycle life. In this study, we introduce a sustainable ethanol-based wet-chemical method to coat NCM111 cathodes with a thin layer of Al2O3, aimed at mitigating surface degradation mechanisms. Beyond studying the coating itself, our study also emphasizes the importance of isolating the benefits of the protective coating from those of re-sintering and surface contaminant removal.
The effects that washing, sintering, and coating have on the electrochemical performance of NCM111 were systematically evaluated. The Al2O3-coated NCM111 demonstrated a substantial improvement in cycling stability, retaining 88% of its initial capacity after 500 charge-discharge cycles, compared to 79% for re-sintered and 65% for untreated samples. While sintering alone enhances cycle life by regenerating the material and eliminating residual lithium compounds on the particle surface, the Al2O3 coating further augments the electrochemical performance, most likely by helping to maintain the layered structure and inhibiting adverse reactions with the electrolyte.
This study presents a cost-effective, environmentally friendly approach to extend the cycle life of NCM cathodes. This method, combined with doping and microstructural modifications, could significantly advance the cycle life of next-generation lithium-ion batteries, especially when applied on high Ni-content NCM materials.
Acknowledgements. The support of the M-Era.net project “Inert Coatings for Prevention of Ageing of NMC Cathode for Lithium-Ion Batteries” (InCoatBat), Project Reference Number 10341 is acknowledged. Institute of Solid-State Physics, University of Latvia as the Centre of Excellence has received funding from the European Union’s Horizon 2020 Framework Program H2020-WIDESPREAD-01-2016-2017-TeamingPhase2 under grant agreement No. 739508, project CAMART2.
P57: The Effect of Filling Strategies on the Surface Morphology of Valve-Jet Printed Cathodes
Esa Hannila (Ph.D. / Postdoc)
University of Oulu, Finland
Energy storage solutions are needed at an ever-growing rate in the modern world. The main driving force is demands for higher efficiency and sustainability for which printing technologies have shown great potential. In this study we use high-speed valve-jetting to fabricate cathodes. Valve-jetting lacks production speed if compared to e.g. screen-printing but offers fast customization and flexibility for production. Due to its modular 5-axis design, it is compatible with a variety of slurries and even three-dimensional structures. The study focuses on the effect of filling strategies by evaluating printed samples through optical and electrochemical battery tests. Based on the results, printed cathodes have similar surface roughness regardless of the filling strategy. However, different strategies have characteristic surface profiles as thicker areas, which could affect the battery performance and lifetime. Thus, process optimization is needed to ensure safe and reliable batteries.
P58: Analyzing the SEI on Commercial Battery Electrodes with Enhanced Raman Spectroscopy
Matthijs Holthuijsen (Ph.D. student)
Beyonder, Norway
This industry PhD project (a collaboration between Beyonder AS and the University of Stavanger) aims to study early-stage solid-electrolyte interphase (SEI) evolution for commercial lithium- and sodium-ion battery chemistries. Raman spectroscopy can be used to study organic and inorganic substances like those found in an SEI, but it generally lacks sensitivity. This problem can be solved by applying nanoparticles to the electrode, a method known as Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy (SHINERS). Gold nanoparticles around 50nm in size resonate with red laser light, strongly boosting the Raman signals of nearby molecules. This signal enhancement is orders of magnitude stronger than signals from the bulk electrolyte, allowing SEI species to be studied with little interference. This method will be used to study the composition of the SEI with different electrolytes and formation protocols, to help predict long-term battery performance at an early stage.
P59: Fluoride-Free Water-Processable Electrodes for Sustainable Batteries
Søren Laurs Lopdrup (Ph.D. / Postdoc)
Aarhus University, Denmark
Worldwide extensive research efforts are made to enhance the sustainability of Li-ion batteries. This entails both the use of abundant and non-toxic raw materials for the battery components as well as benign chemicals used in the production processes. For the battery electrodes, substantial progress has been made for the redox active materials, however, the polymeric binder materials used in the electrodes are still an issue that has not been widely addressed up to now.
The binder plays an important role in battery electrode by maintaining the mechanical cohesion of the active material with the conductive agent (carbon) and with the metallic foil of the current collector throughout the lithiation and delithiation processes. The common binder used in Li-ion battery electrodes is Polyvinylidene fluoride (PVDF), and N-Methyl-2-pyrrolidone (NMP) is used as solvent for electrode production1,2. Herein, we present a novel fluoride-free binder which is processable in water, which nat-urally enhances the sustainability of the electrode significantly. The polymeric binders herein are syn-thesized via a homopolymerization and used as binder in the LiMn1.5Ni0.5O4 (LMNO) cathode composition.
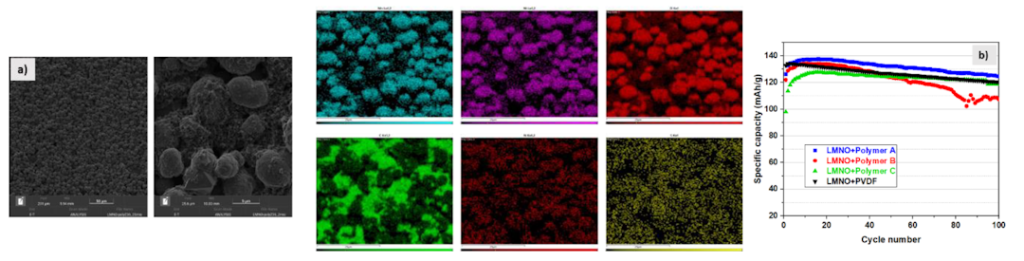
P60: Battery Lifetime Estimations for Freight Train on Partially Electrified Railway Route
Jonathan Fagerström
Institute for Energy Technology (IFE), Norway
The railway sector is considering the implementation of partially electrified routes, which would involve the use of both electric catenaries for charging and “off-grid” battery propulsion for locomotives. Such a set-up enables multiple system designs for cost-efficient and reliable operation. The purpose of this work was to generate realistic drive cycles for a freight train and analyze how battery lifetime is affected by system design. Two different battery types were included, and the battery capacity was varied to study how different stress factors impact the battery lifetime. Using an empirical battery degradation model, tuned for a power cell of NMC pouch type, it was found that a low-capacity battery pack loses 5% more capacity compared to a high-capacity battery pack. It was also found that 500 return trips reduced the low-capacity pack to 85% of rated capacity. These findings will be compared with degradation results of a cylindrical LFP power cell.
P61: Innovative Hard Carbon Materials for High Energy Sodium-Ion Battery Applications
Subramani Kaipannan (Postdoc.)
Norges teknisk-naturvitenskapelige universitet (NTNU), Norway
Hard carbon (HC) derived from biomass materials is gaining attention as a sustainable and cost-effective anode material for sodium ion batteries (SIBs) due to its potential to reduce reliance on fossil precursors and lower environmental impact. This study focuses on the development of HC from sawdust of Norway Spruce (Picea abies Pinales) and performance evaluation as an anode material for SIBs. We explore the impact of different synthesis methods on the structural and electrochemical properties of HC, aiming to optimize its particle size, surface area, and porosity making them suitable for commercial SIBs. Electrochemical testing, including cyclic voltammetry, and galvanostatic charge-discharge cycles, demonstrates that our HC anodes exhibit high capacity, high rate, and excellent cycle stability. These findings highlight the potential of HC as a viable anode material for high-performance SIBs, enabling sustainable energy storage.

P62: Urea Etching of Si Microparticles for Li-Ion Battery Anodes
Ali Abo Hamad (Ph.D. student)
Mid Sweden University, Sweden
Silicon-based anodes offer high capacity for lithium-ion batteries (LIBs) but face challenges due to volume expansion. The study introduces a novel approach using urea as a dual etchant for silicon microparticles. This process involves mechanical etching via urea phase transfer within silicon voids and chemical etching induced by urea and ammonia gas. Elevated temperatures trigger urea decomposition, generating ammonia gas and solid by-products. Material characterization confirms the formation of cracks, voids, and pores on silicon microparticles. Two temperature programs (220°C and 400°C) are employed, each addressing solid by-product removal differently. Integrating porous silicon particles into anodes with graphite enhances capacity and cycle stability. With 9% porous Si content, the initial capacity reaches 660 mAh/g, maintained at 520 mAh/g after 150 cycles at 100 mA/g. This research sheds light on dual etching mechanisms with urea, enhancing silicon-based anodes in LIBs.
P63: All Solid-State Thin Film Li-ion Batteries as Tool to Study Binary Si-based Anodes
Nienke Visser (Ph.D. / Postdoc)
Mid Sweden University, Sweden
Silicon is regarded a promising candidate to supplement or substitute graphite as anode material in LIBs, due to its high lithium storage capacity. Its large volumetric change during cycling is problematic, as it causes a significant capacity fade. The use of binary Si alloys, in which Si is combined with active or inactive materials, such as Sn, Al or Fe, can help to increase the stability of the anode material. Although material screenings have shown positive impacts on electrochemical and cyclability performance of binary Si-based anodes, detailed knowledge on the stabilisation mechanisms is lacking.[1] We use physical vapor deposition (PVD) to prepare all solid-state batteries consisting of Si alloys (anode), LiPON (electrolyte) and LCO (cathode). These thin film batteries are ideal for an operando TEM study, with which we aim to probe the mechanism of (de)lithiation of binary Si anodes to better understand their stabilisation mechanism.
[1] J. Power Resources, 520 (2022) 230871
P64: A Direct Comparison of a Power and an Energy-Optimized Li-ion Pouch Cell: Characterization, Ageing and Modelling
Julia Wind (Researcher)
Institute for Energy Technology (IFE), Norway
Depending on the application, LIBs are normally optimized for either supplying as much power as possible or storing as much energy as possible. A power optimized cell typically has lower energy density and thinner electrodes, compared to an energy optimized cell with higher energy density and thicker electrodes. However, increased electrode thickness leads to several challenges and affects cell performance and ageing characteristics.
In this study we aim to develop a fundamental understanding of electrode performance and limitations through the evaluation of high-energy and high-power electrodes from state-of-the-art LIBs. For this purpose, we selected two large-scale commercial pouch cells with similar geometry, chemistry, and capacity: one optimized for power and one for energy.
Both cell types were tested at different temperatures, currents and SoCs. Cell-tear-down analysis revealed differences in battery design. The results were used to parameterize a P2D electrochemical model.
P65: Synthetic Graphites as the Cathode Material for Aluminium-Carbon Batteries
Helene Lillevestre Langli
Norges teknisk-naturvitenskapelige universitet (NTNU), Norway
P66:
Kristian O. Sylvester-Hvid
DTU Energy, Denmark
P67: On the Moisture Tolerance of LiFSI-based Lithium-Ion Batteries: A Systematic Study on NMC622/Graphite Cells
Weldejewergis Kidanu (Postdoc)
Norges teknisk-naturvitenskapelige universitet (NTNU), Norway
Due to its electrochemical stability, ionic conductivity and passivation of the Al current collector, state-of-the-art LiBs mainly use LiPF6 as an electrolyte salt. However, its thermal instability, sensitivity to trace amounts of moisture and the consequent formation of HF need to be addressed with alternative salts such as LiFSI. On the other hand, during LiB cell manufacturing, 80~95% of the energy consumption occurs in electrode drying and dry room conditioning. Hence, LiFSI-based LiBs that can tolerate certain levels of moisture are of interest to reduce the energy consumption.
Herein, effect of moisture on LiFSI based NMC622/Li and graphite/Li half and NMC622/graphite full cells were investigated, and surface chemistry and morphology of cycled electrodes were characterized with XPS and SEM. It was found that ~1000 ppm H2O slightly improves the capacity retention of the NMC622 half cells; however, severely degrades the graphite half-cell and NMC622/graphite full cells.