Rational Design and Controllable Synthesis of SiOx Anode Materials from Diatom-SiO2 Nanostructures for Lithium-Ion Batteries
Kesavan Thangaian
Functional Materials and Materials Chemistry (FACET), Department of Materials Science and Engineering, Norwegian University of Science and Technology
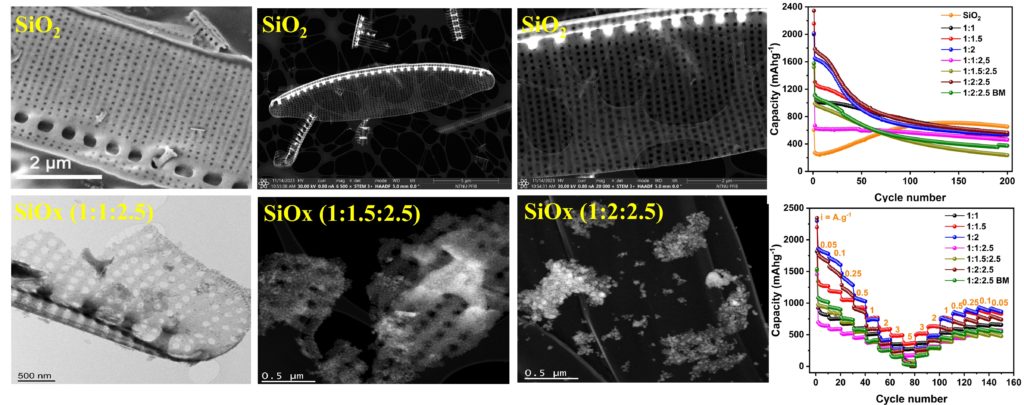
We have demonstrated the rational design and controllable synthesis of three different compositions of SiOx by varying the ratios of SiO2:Mg:NaCl in the reduction process. The electrochemical performance of SiOx was investigated as an anode material for LIBs. However, the electrochemical performance of SiOx is affected by several structural parameters, such as chemical composition, particle size, surface area, and crystallinity. XRD pattern confirms the highly crystalline pure phase formation of Si, and microscopic images reveal that the morphology of the original frustules preserved with pores is seen visibly on the surface. The SiOx anodes exhibited a high specific capacity, and it was found that the electrochemical properties of SiOx mainly depended on the ratio of Si to SiO2 in the structure. SiOx (SiO2:Mg:NaCl = 1:2:2.5) delivers 563 mAh.g-1 after 200 cycles at 100 mA.g-1. Diatoms SiO2 and SiOx prepared without NaCl have been subjected to the same cycling protocol for comparison.